New lithium battery ditches solvents, reaches supercapacitor rates
The all solid-state battery operates between -30 and 100 degrees Celsius.
by John Timmer - Mar 22, 2016 3:40am SAST
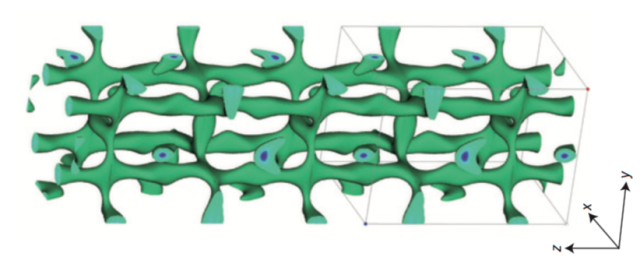
The distribution of lithium ions within the new electrolyte material provides obvious routes for the ions to move between electrodes.
Kato et. al., Nature Energy
For most of us, the only way we judge a battery is by how long it can deliver electrons to our favorite devices. But many applications require more than that. They need batteries that operate across a large temperature range, are compact or flexible, and can manage a fast charge/discharge rate. Plus, we'd all like them not to explode or fail suddenly.
Most energy storage tech involves balancing a trade-off among these various properties. But a new report from a collaboration between academic researchers and Toyota seems to promise it all: a battery more compact than lithium-ion, a better energy density, the charge speed of a supercapacitor, and improved safety. How is this all possible? They got rid of the liquid electrolyte typical of most lithium-ion batteries.
In principle, batteries are structurally very simple: two electrodes where ions exchange electrons, separated by an electrolyte that allows the ions to shuffle between the two. These electrolytes are almost always liquids, since they can easily dissolve the ions, allowing their free movement between the electrodes. Unfortunately, leaking electrolytes are often a cause of failure in these batteries. Fixing this is a challenge—you can't just shove ions through a solid, right?
Actually, you can, just not as efficiently. A few solid-state electrolytes have been developed, but they haven't had the performance of the existing technology on the market, largely because of the difficulty of pushing ions across the solid electrolyte. But a paper involving Toyota researchers, published in 2011, showed that some solids can be pretty good at conducting lithium ions. The trick is that these solids need to have a crystal structure that arranges the lithium ions in a line, essentially creating a channel for them to move along.
The problem is that all of the solids they identified with this structure were either chemically unstable or involve expensive raw materials. But in the intervening years, the authors have focused on testing other material and found a couple with some rather impressive properties.
One of them is a crystal with the rather complex formula of Li9.54Si1.74P1.44S11.7Cl0.3. This turned out to be very effective for high current applications. Another material, Li9.6P3S12, was better for high-voltage cells. Both of them were chemically stable, and neither involved any expensive raw materials. The authors determined their structure, which revealed that the new materials provided a three-dimensional grid, allowing ions to move in nearly any direction.
This may in part explain the new materials' improved properties, which include a conductivity twice that of their earlier solid electrolyte.
The authors built batteries that used these solid-state electrolytes and showed that the batteries could operate within a huge temperature range: -30 degrees Celsius up to 100 degrees Celsius. Commercial lithium-ion batteries don't function at either of the extremes of this temperature range. They also had a very fast charge/discharge capability, being able to complete a charge/discharge cycle in under seven minutes. At high temperatures, discharge rates were competitive with supercapacitors. The energy density (a weakness of supercapacitors) turned out to be competitive with that of lithium-ion batteries.
Because chemical reactions occur between the electrodes and this material when it is first run through a charge/discharge cycle, it loses some 10 percent of its capacity right at the start. But, after 500 cycles, it still retains 75 percent of the original capacity, indicating losses after that first cycle are relatively small.
Why is this solid electrolyte so high performing? It's structured so that it provides natural avenues for the lithium ions to travel along, and its three-dimensional mesh allows them to bypass any defects. And, within the solid, there are simply more lithium ions per unit volume than you can possibly get in a solution—over 20 times as many. Because the solid can't freeze and won't chemically degrade at high temperatures, it has a much higher operating range. Plus, it won't leak anywhere.
Does this solve all our battery problems? Absolutely not. Both the electrode materials still matter, as they have to tolerate large changes in their lithium content without swelling or shrinking so much that they cause structural failures. (The authors indicate that they've only started testing different electrode materials and hope to build even better batteries with the right combination.) And having fast charge/discharge cycles requires delivery of all the electricity involved in the recharging, which poses its own challenges.
Still, the new material could cut down on some of the common points of failure that liquid electrolytes bring. And the authors argue that this in turn would help simplify the batteries structurally, allowing a greater overall energy density. There are some experimental technologies, like lithium-air and lithium-sulfur, that can outperform these. But the solid electrolyte may allow a well-understood technology to keep up with those alternatives.
Nature Energy, 2016. DOI: 10.1038/NENERGY.2016.30 (About DOIs).
source: http://arstechnica.com/science/2016...itches-solvents-reaches-supercapacitor-rates/